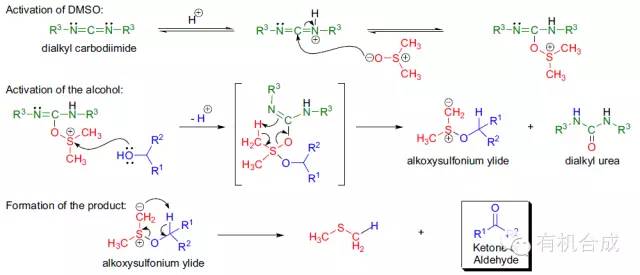
通过DCC和DMSO氧化醇得到醛酮的反应,也被称为Pfitzner-Moffatt氧化。反应中常常加入布朗斯特酸催化反应反应。与斯温氧化或Parikh-Doering氧化所不同的是,活性因子分子体积较大而容易受到空间位阻的影响。利用到这一特点,反应能够选择性氧化空间位阻较小的醇。另外,室温条件下反应能够进行也是其一大优点。作为副产物生成的尿素比较难除去,以及副反应硫醚化较容易发生也是反应的一个不足之处。
包括以下几步:1、DMSO被质子化的二烷基碳二酰亚胺活化;2、醇的活化和烷氧基硫叶立德的生成;3、烷氧基硫叶立德的内部重排生成相应的醛酮和副产物二烷基脲。所有的活性DMSO 的氧化中,硫叶立德都是必要的中间体。
反应特点有:1、反应条件温和,不会过氧化,试剂易得,操作简便;2、小试和放大反应的产率都较高;3、很少有副反应,有时会生成甲基甲巯基醚和β,γ不饱和羰基化合物的异构化;4、反应的官能团耐受度很好,但叔醇可能会发生消除;5、DCC是最常用的的活化试剂,反应中一般会加过量(≥3eq);6、DMSO要六倍当量以上,可以做溶剂,但加入一些惰性的混合溶剂(乙酸乙酯,苯)有利于产品的分离;7、要求温和的酸催化条件,如正磷酸,二氯乙酸和吡啶的强酸盐等等,酸一般加0.5eq产率最高,在强酸条件下,会阻止硫叶立德的生成,不能进行反应或反应很慢;8、反应会生成二烷基脲,不容易除去,可也用一些水溶性的碳二酰亚胺试剂可以解决,如EDC,另外过量的DCC在处理时可以加入草酸除去。
反应的常用操作步骤:
General procedure for oxidation ofalcohols by Pfitzner–Moffatt method
3 equiv. of a carbodiimide are addedover a solution of 1 equiv. of the alcohol and 0.5 equiv. of pyridiniumtrifluoroacetate in 0.6–40 mL of neat dry DMSO or a mixture of DMSO andbenzene, at room temperature. When most of the starting compound has beenconsumed, the work-up can be made according to the following alternatives:
Work-up A:
The solvent is removed at the rotaryevaporator, and the resulting residue is purified by chromatography. It can beadvisable to filter the precipitate of DCU-formed when DCC is used-beforeremoving the solvent. In order to avoid interferences from unreactedcarbodiimide, it can be advisable to transform it in the corresponding urea bycareful addition of oxalic acid-either solid or in a solution in methanol- tothe stirred reaction mixture. Addition of oxalic acid produces a copiousevolution of gas that signals the duration of the hydrolysis of thecarbodiimide.
Work-up B:
The reaction mixture is fractionedbetween water and an organic solvent, such as diethyl ether, ethyl acetate ordichloromethane. The organic phase is sequentially washed with water and withan aqueous solution of NaHCO3, dried with Na2SO4or MgSO4 and concentrated. When DCC is used, the resulting residuewill contain unreacted DCC and DCU that will need to be separated bychromatography.

【J. Am. Chem. Soc. 1992, 114, 1438-1449.】

【Liebigs Ann. Chem. 1989, 939-943】


【J. Am. Chem. Soc. 2006, 128, 3926.】
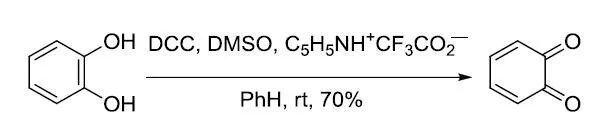
【Synthesis 1987, 741-742】
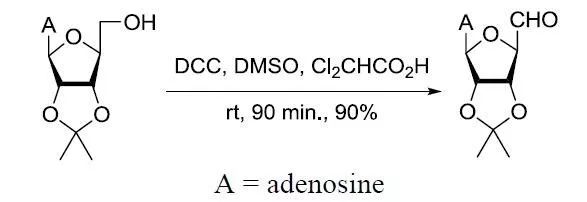
【J. Med. Chem. 2005, 48, 3649-3653】
【J. Org. Chem. 2011, 76, 2-12】
化学慧定制合成事业部摘录