1990年Umemoto报道了化合物1和2的合成及应用,这是第一种亲电性的三氟甲基化试剂【Umemoto, T; Ishihara, S. TetrahedronLett. 1990, 31, 3579】。随后Umemoto报道了化合物3和4的合成及应用【(a) Umemoto, T; Ishihara, S. J. Am. Chem. Soc.1993, 115, 2156. (b) Umemoto, T; Ishihara, S; Adachi, K.J. Fluorine Chem.1995,74, 77】。
这几个化合物是稳定的晶体,具有良好的稳定性。苯并环是很好的离去基团,在取代过程中容易离去,有利反应进行。反应易于处理,特别是化合物4,反应生成后的磺酸是水溶性的,极易除去。这种三氟甲基化并不是通过CF3+进行,也不是SN2取代,可能是通过SET机理产生三氟甲基自由基,然后对碳负离子的亲电加成。
这种方法通用型强,通过这种方法,可以在多种亲核性化合物(anions of b–diketones, b–keto esters and malonates, acetylideions, silyl enol ethers, enamines, activated aromatics, heteroaromatics,alkane- and arenethiolate anions, halide anions, and various enolate anions)上引入三氟甲基。但这几个试剂制备困难,价格较高,限制了其应用。
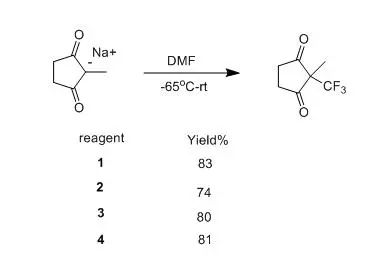
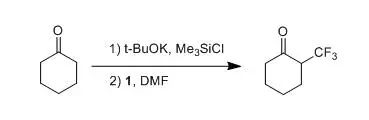
【Umemoto, T; Ishihara, S.TetrahedronLett.1990,31, 3579】
【Umemoto, T; Ishihara, S.J. Am. Chem. Soc.1993,115, 2156】
【Umemoto, T; Ishihara, S; Adachi, K.J. Fluorine Chem.1995,74, 77】
Under an argon atmosphere, to a solution of Ethynyl-benzene (1.02 g, 10 mmol) was dropped n-BuLi (2.5M, 6 mL, 15 mmol) at –78oC, the mixture was stirred for 1 h, trifluoromethyl onium salt 3(11 mmol) was added in several portions. Thereaction mixture was stirred for another 1 h at room temperature, then quenchedby saturated NH4Cl solution, and extracted with EA. The combined organic layer was dried over Na2SO4, concentrated under reduced pressure. The residue was purified by column chromatography onsilica gel to give the desired product (0.58 g,58%).
【Umemoto, T; Ishihara, S.J. Am. Chem. Soc.1993,115, 2156】
化学慧定制合成事业部摘录







