反应机理
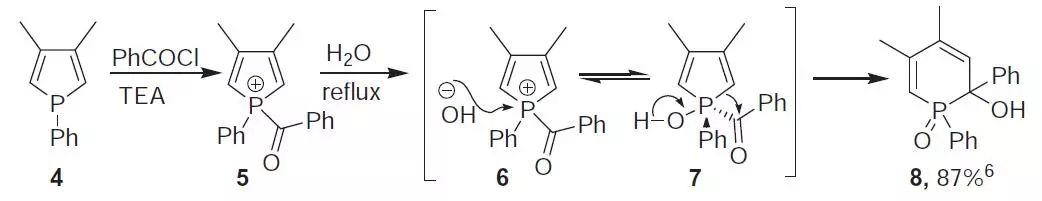
反应实例
9-Methyl-9,10-dihydro-9-phosphaphenanthrene-9-oxide (3).1 The phosphonium salt 2 (R = Me, 0.7 g, 1.5 mmol) in aq acetone containing KOH solution was heated to reflux for 2 h. Extraction of the cold mixture with CHCl3, evaporation of the solvent and silica gel chromatography via elution with EA:EtOH (7:3) afforded 0.24 g, 71% of 3.
【Allen DW, Millar IT, Chem Ind, 1967, 2178】
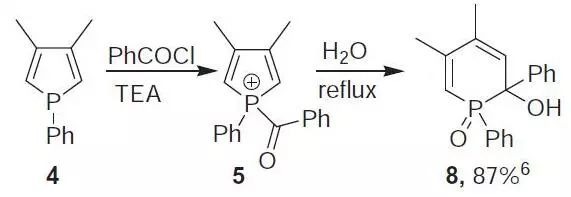
Hydroxyphosphine oxide (8).6 Benzoyl chloride (10 g, 71.1 mmol) was added to 4 (7.53 g, 40 mmol) and Et3N (20 mL) in Et2O (300 mL). After 3 h stirring under reflux 5 was hydrolyzed with water (150 mL) for 2 h. The precipitates thus formed were removed by filtration and the resulting filtrate dried over MgSO4. Evaporation of the solvent and recrystallization from PhCH3 afforded 10.8 g of 8 (87%).
【Mathey F, Tetrahedron,1973, 29, 707】
相关文献
1, Allen DW, Millar IT, Chem Ind, 1967, 2178
2, Trippett S, Chem Comm, 1967, 1113
3, Allen DW, Millar IT, J Chem Soc C, 1969, 252
4, Tebby JC, J Chem Soc C, 1971, 1064
5, Mathey F, Tetrahedron, 1972, 28, 4171
6, Mathey F, Tetrahedron, 1973, 29, 707
7, Allen DW, J Chem Soc Perkin 1, 1976, 2050
8, Markl G, Angew Chem Int, 1987, 26, 1134
9, Keglevich Gy, J Org Chem, 1990, 55, 6361
10, Keglevich Gy, Synthesis, 1993, 931
11,R Savignac P, Eur J Org Chem, 2000, 3103
12, Mapp AK, J Am Chem Soc, 2006, 128, 4576
13, Vignolle J, Tet Lett, 2007, 48, 685
编译自:Organic Syntheses Based On Name Reactions, 3RdEd, A. Hassner, Page 7-8。







